Orthognathic Surgery
OSA
OMS and Other Dental Specialists
Session: Orthognathic and OSA Track: Hot-off-the-Press and Abstract Session
GP2d - One-Year Outcomes of Selective Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea: A Single Center Clinical Experience With 100 Cases and Current Challenges for Oral and Maxillofacial Surgery
Thursday, September 12, 2024
4:05 PM - 4:15 PM EDT
Location: Tangerine Ballroom WF1, Orange County Convention Center

Minsung Kim, DDS (he/him/his)
Resident
Christiana Care
Wilmington, Delaware- EP
Etern Park, MD, DDS
Attending Surgeon
Christiana Care
Wilmington, Delaware
Oral Abstract Presenter(s)
Co-Author(s)
Abstract: Hypoglossal nerve stimulation is an increasingly widespread treatment for obstructive sleep apnea (OSA) in those who cannot tolerate continuous positive airway pressure (CPAP). Since selective hypoglossal nerve stimulation was approved by the FDA in 2014, approximately 52,000 cases were performed in the United States. Hypoglossal nerve stimulation activates upper airway dilator muscles, including the genioglossus and has been shown to reduce OSA severity. It helps to prevent upper airway collapse by delivering an electrical stimulation to advance the tongue musculature with each inspiration. Initial criteria for hypoglossal nerve stimulation are age 18 or over, diagnosed with moderate to severe OSA, unable to tolerate CPAP, BMI of 32 or less.The object of this study is to evaluate the safety and efficacy of selective hypoglossal nerve stimulation for treatment of OSA at 12 months following implantation. At Christiana Care Health system, 244 patients with moderate to severe OSA with positive airway pressure intolerance underwent surgical implantation and activation. Of those, 100 patients had at least one year follow up with successful fine tuning. There was significant improvement from baseline to 12 months in apnea-hypopnea index (AHI). Average preoperative AHI was 34.2 and postoperative AHI was 5.7. Overall, 83% AHI reduction was observed. Of 244 cases, 1 patient required explant due to delayed hardware infection. No major perioperative complication was reported. Hypoglossal nerve stimulation is relatively less invasive, which reduces recovery time and complications as compared to other surgical modalities for OSA treatment, such as, maxillomandibular advancement and uvulopalatopharyngoplasty. The surgical approach has been modified from 3 incisions to 2 incisions. The new approach, which was approved by FDA in 2021, provides a shorter operative time without an increase in complications as compared to the 3-incision approach. Despite the high success rate of selective hypoglossal nerve stimulation, very few cases are being offered by oral and maxillofacial surgeons. Medicare has prohibited oral and maxillofacial surgeons from providing hypoglossal nerve stimulation. Hypoglossal nerve stimulation is an effective alternative for oral and maxillofacial surgeons to expand the scope of OSA treatment. Currently, the constraints imposed by the insurance guidelines limit our ability to provide much needed care for our patients. A more streamlined and consistent set of insurance coverage guidelines would enable oral and maxillofacial surgeons to broaden the armamentarium of OSA treatment and delivering higher-quality care to our patients.References1. Woodson BT, Soose RJ, Gillespie MB, Strohl KP, Maurer JT, de Vries N, Steward DL, Baskin JZ, Badr MS, Lin HS, Padhya TA, Mickelson S, Anderson WM, Vanderveken OM, Strollo PJ Jr; STAR Trial Investigators. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial. Otolaryngol Head Neck Surg. 2016 Jan;154(1):181-8. doi: 10.1177/0194599815616618. Epub 2015 Nov 17. PMID: 26577774.2. Bellamkonda N, Shiba T, Mendelsohn AH. Adverse Events in Hypoglossal Nerve Stimulator Implantation: 5-Year Analysis of the FDA MAUDE Database. Otolaryngol Head Neck Surg. 2021 Feb;164(2):443-447. doi: 10.1177/0194599820960069. Epub 2020 Sep 22. PMID: 32957866.3. Sagalow ES, Rodin J, Estephan L, Jackson J, Crippen M, Boon M, Huntley C. Two-Incision Approach for Hypoglossal Nerve Stimulator Placement: A Single Institution Assessment. Laryngoscope. 2022 Aug;132(8):1687-1691. doi: 10.1002/lary.30050. Epub 2022 Feb 11. PMID: 35147978.4. Centers for Medicare & Medicaid Services. (n.d.). Local Coverage Determination (LCD): MolDX: Genetic Testing for Inherited Diseases (L38310). [Webpage]. Medicare Coverage Database. Retrieved from https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=38310
Table 1. Selective Hypoglossal Nerve Stimulation One-Year Outcomes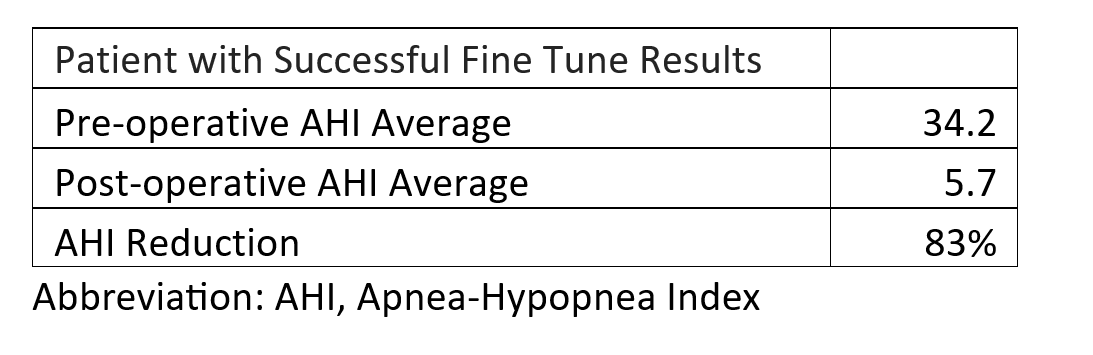
Table 1. Selective Hypoglossal Nerve Stimulation One-Year Outcomes

